-
On the Cooling of Electronics with Nanofluids
W. Escher, T. Brunschwiler, N. Shalkevich, A. Shalkevich, T. Bürgi, B. Michel and D. Poulikakos
Journal of Heat Transfer, 113 (5) (2011), p51401


DOI:10.1115/1.4003283 | unige:15944 | Abstract | Article HTML | Article PDF
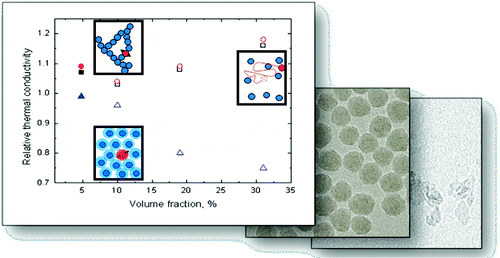
Nanofluids have been proposed to improve the performance of microchannel heat sinks. In this paper, we present a systematic characterization of aqueous silica nanoparticle suspensions with concentrations up to 31Â Â vol %. We determined the particle morphology by transmission electron microscope imaging and its dispersion status by dynamic light scattering measurements. The thermophysical properties of the fluids, namely, their specific heat, density, thermal conductivity, and dynamic viscosity were experimentally measured. We fabricated microchannel heat sinks with three different channel widths and characterized their thermal performance as a function of volumetric flow rate for silica nanofluids at concentrations by volume of 0%, 5%, 16%, and 31%. The Nusselt number was extracted from the experimental results and compared with the theoretical predictions considering the change of fluids bulk properties. We demonstrated a deviation of less than 10% between the experiments and the predictions. Hence, standard correlations can be used to estimate the convective heat transfer of nanofluids. In addition, we applied a one-dimensional model of the heat sink, validated by the experiments. We predicted the potential of nanofluids to increase the performance of microchannel heat sinks. To this end, we varied the individual thermophysical properties of the coolant and studied their impact on the heat sink performance. We demonstrated that the relative thermal conductivity enhancement must be larger than the relative viscosity increase in order to gain a sizeable performance benefit. Furthermore, we showed that it would be preferable to increase the volumetric heat capacity of the fluid instead of increasing its thermal conductivity.